Table 14: Action of Koji on Maltose
Brown and Heron have shown that when an aqueous solution of malt is allowed to remain in contact with a solution of cane sugar, a change takes place by which the cane sugar is made to take up water and is thereby converted into invert sugar, a mixture of dextrose and levulose. The diastase of malt is said by them to exert its maximum effect upon sugar at 55°C; its action is considerably weakened at 60°C and almost destroyed at 66°C.
Experiment shows that the extract of koji also possesses the property of causing cane sugar to become inverted, but I am not able to define the limits of its action. The following experiments will suffice to prove this point.
Experiment 1: 1.974 gram of dry cane sugar was dissolved in 25 cc of koji extract, then diluted with water to 100 cc. The amount of rotation was found to be 15.8 divisions, and the calculated number 15.5 divisions.
|
1.974 grams case sugar dissolved in 100 c.c. gives rotation
|
= 12.1 div.
|
|
25 c.c. koji solution diluted to 100 c.c. gives rotation
|
= 3.4
|
|
|
15.5 div.
|
|
Unaltered cane sugar (0.7294 grams in 100 c.c.)
|
+4.4 div.
|
|
Koji extract (25 c.c.) in 100 c.c. of water)
|
+3.4 div
|
|
Invert sugar formed
|
-2.37 div.
|
|
|
+5.43 div
|
Calculated in degrees of arc the specific rotatory power of the can sugar has been reduced from 74° to 10°.
|
|
Optical rotation
|
Specific rotatory power
|
|
At starting
|
24.8 div.
|
74°
|
|
After 1-1/2 hours at 15°C
|
23.7
|
70.6
|
|
After 20-1/4 hours at 10-12°
|
21.0
|
62.6
|
|
After 3/4 hour more at 40°C
|
20.2
|
60.2
|
|
|
Optical rotation
|
Specific rotatory power
|
|
After 1-1/4 hours at 40°C
|
11.2 div.
|
50°
|
|
After 2 hours at 45-50°
|
4.0
|
17.8
|
The experiment was not carried further than this. At low temperature the converting action of this particular extract of koji is very slow, but at higher temperatures, and especially at from 45° to 50°C it is much more rapid. In this respect, therefore, koji extract resembles malt extract.
Action Upon Maltose
So recently as 1872 Mr. O'Sullivan1 directed attention to the nature of the sugar formed when malt extract is made to act upon gelatinized starch, and his experiments conclusively established the existence of a new sugar, previously, however, pointed out by Dubrunfaut, which is now known as maltose. In composition it agrees with cane sugar, but differs from it in having a specific rotatory power of 150°, and in forming dextrose and not invert sugar when boiled with acids or otherwise hydrated. It also differs in its reducing action upon oxide of copper from either cane sugar or dextrose, for the former has no reducing action upon cupric oxide, while maltose reduces only 61 to 63% of the amount reduced by the same weight of dextrose. Messrs. Brown and Heron2 have shown that a solution of malt is not able to convert maltose into dextrose, and that it is quite without action upon it. The following experiments will, however, show that the solution of koji possesses the property of hydrating maltose and converting it into dextrose. This will be rendered evident by the change which the solution of maltose undergoes under the influence of koji extract both as regards the weight of oxide of copper reduced by a given weight of the solid, and as regards the specific rotatory power of the product.
The maltose employed was obtained from ame, a kind of sweetmeat prepared by the action of malt in solution upon the starch contained in millet or in rice. Various specimens of ame contained from 68 to 94% maltose, which was separated according to the process described by O'Sullivan3. The specimens employed were in the crystalline state, and contained water sufficient to reduce the specific rotatory power from 150° to 144.5°.
The specific rotatory power therefore, fell from 144.5° to 77.6° in 2 hours, and would doubtless have fallen to 59° if the solution had not been used up after 2 hours. The action may be represented in the form of a curve using time and specific rotatory power as abscisse and ordinates respectively (See Figure 3).
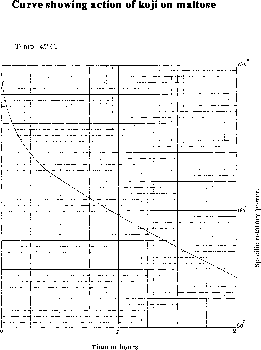
The curve shows very clearly how regular the action is, and leaves no doubt about the power of extract of koji to effect the hydration of maltose. It is especially important to establish this, because this property marks in the sharpest manner the difference between malt extract and koji extract. Brown and Heron's experiments leave no doubt about the inability of malt extract to convert maltose into dextrose, and these experiments, I think, establish conclusively the ability of koji extract to do this.
Action Upon Dextrin
The action of koji upon dextrin is to cause it slowly to combine with water and form dextrose, as the following experiment shows.
2 Jour. Chem. Soc. 1879. Trans., p. 621.
3 Journ. Chem. Soc. 1876, ii, p. 127.
stevens@stsci.edu