[Top] [Prev] [Next] [Bottom]
Chapter 10. Preservation of Sake
Clearing
The liquid which has passed through the press is turbid and requires clarification before being used. This is effected by collecting the sake in large tuns which have two holes near the bottom one above the other, and closed by means of plugs (see Figure 1). After the lapse of about 15 days the suspended matter has settled to the bottom, and the greater part of the clear liquid may then be drawn off by removing the upper plug, and collecting the liquid in proper vessels. The remainder is allowed to stand for a longer time, and the clear part is separated by opening the lower hole. What remains is termed ori and is added to another brew just before filtering.
Heating
The clear sake so produced would not keep for more than a few days in the warm weather without being subjected to some further process. At Itani and at Nishinomiya the heating of the sake is carried out on the 88th night called hachiju-hachiya, which usually occurs between the 24th and 25th of the fourth month of the old calendar. The operation is a very simple one. A large iron pan is built in the ground, so that the upper part is only about 5 or 6 inches above the surface; on one side the ground is cut away, and a fire-place arranged below the pan, the opening being some distance below the floor. The bottom of the pan is heated directly by the flame from a wood fire, and the heating is continued until the liquid is so hot that a workman can just dip in his hand three times in succession without feeling much inconvenience. At some works thermometers have been introduced, and the temperature indicated varies from 120°F to 130°F. Whilst still hot the sake is transferred to the store vats, large tuns holding about 40 koku, made of sugi (cryptomeria japonica) or hinoki (chamaecyparis obtusa). They are closed by lids and the interval pasted round with paper fastened by means of a kind of glue made from seaweed (funori). In these tuns the liquid will keep without alteration so long as the weather remains cold, but as soon as the summer sets in, the sake has to be frequently examined in order to detect any change. When any signs of alteration are apparent it has to be taken out of the tun, and again heated, after which it is returned to the store vat.
In the following table (Table 26) are given analyses of several kinds of sake obtained from the districts of Itami and Nishinomyia. They were in most cases obtained directly from the respective brewers, and may be regarded as pure and unadulterated samples.
The samples of sake of which analyses are given in the table were brewed in the winter of 1879-80, and had been subjected to the operation of heating only once. As will be seen there is not a very great variation in their composition, the percentage of alcohol not passing beyond the limits 11 to 14. The quantities of dextrose and dextrin are very small, and in this circumstance, as well as in the larger percentage of alcohol, lies the essential difference between sake and beer. Connected with the absence of the two latter bodies also is the freedom from carbonic acid, for the sake is quite as still as the most fermented wine. When newly prepared it possesses a pale straw color, and has a peculiar unripe taste, but on keeping, and especially after heating, the color darkens and the taste becomes more matured. During the hot weather it is impossible to prevent the sake "turning" without frequent heating, and as this is a very laborious operation any improvement would be welcomed by the brewer. Several samples which have undergone this change have been examined; it appears to be accompanied by the formation of butyric acid, ammonia, and a volatile, ill-smelling substance, whilst at the same time a portion of the alcohol disappears. One sample which had been allowed to stand from the spring of 1879 until June 1880 contained 11.4% of alcohol and 0.316% of total acid, of which 18% was butyric acid. As the original sake was not completely analyzed I cannot give it for comparison. Two samples of those already given were kept and analyzed after standing in bottles corked in the usual way from February 5th, 1880, until January 17th, 1881, and November 1st, 1880, respectively. The composition of the original sake is repeated alongside for convenience and comparison.
In both cases a diminution in the percentage of alcohol took place after keeping, and at the same time the small quantity of dextrose present in the original sake disappeared. The principal apparent change is the large increase in the percentage of fixed acid, whilst at the same time a small quantity of butyric acid is also formed. It is the presence of this acid together with the volatile body before mentioned which causes the disgusting smell possessed by such "turned" sake, notwithstanding the very small percentage contained in the liquid, but the sour taste of spoilt sake is due to the fixed acid, mainly lactic acid. The quantities of alcohol and dextrose which have disappeared are much greater than the weights of acid formed, a circumstance which shows that a combustion occurs resulting in the formation of carbonic acid and water. In fact the liquid which has been kept over a summer will be found to be highly charged with carbonic acid, whereas unaltered sake contains none.
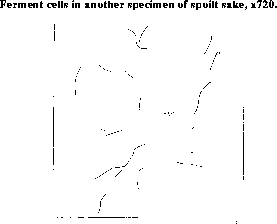
At the bottom of the vessel in which the sake has been kept is a thick deposit consisting almost entirely of minute cells some of which are represented in Figure 25 and Figure 26. The organisms in Figure 25 resemble those found in putrid beer, and those in Figure 26 are almost identical with the filaments which produce "turned" beer. The former are more commonly met with, and doubtless, by their growth give rise to the unpleasant odor characteristic of spoilt sake. It is for the purpose of destroying these organisms that the sake is heated, but as, after heating, no precaution is taken to prevent the contact of the liquid with fresh germs, repeated heatings are necessary. Indeed during the hot months from June to September, the sake must be heated at least once a month and very often more frequently.
It is an important and interesting fact that the process of heating the sake for the purpose of preserving it has been in use in Japan for about 300 years, and it is all the more remarkable, that having discovered the beneficial effect of this operation, the brewer should not have made it lasting by taking precautions against subsequent contaminations. Instead of doing this, however, the liquid after having been heated is returned to the same store vats in which it was formerly kept, and the sides of which still retain particles of the ferment attached. When the still hot liquid is put into the vat, it is possible that the high temperature will kill all those germs adhering to the sides of the tun so far as the liquid rises. But above the level of the liquid they will remain untouched, and as, during the subsequent standing of the sake, the alcohol is drawn up the sides of the tun and runs back again in the form of "tears", the germs will in that way be carried down into the sake, will slowly develop, and in a comparatively short time will render it undrinkable.
The Japanese brewer has been credited with the discovery of the method of preserving alcoholic liquids which has made the name of M. Pasteur so widely known, but when we consider that in Japan, the heated liquid is allowed to become inoculated with the germs of its disease, even at the time of tis so-called preservation, we see that he has omitted a part of the process which Pasteur truly regards as vital. When an alcoholic liquid has been "pasteurized," as the expression is, it will keep for an indefinite time, because the germs of disease which were already present have been killed by the high temperature of the liquid, and care is subsequently taken that no fresh germs find access to it. A wine thus treated not only does not deteriorate but actually improves by keeping, because it is allowed to "age" without the danger of any malady being set up which would spoil it. The Japanese wine, sake, is not allowed to improve in this way; I have in vain endeavored to get samples which have been preserved for several years. As a rule, even at the most extensive breweries in Itami and Nishinomiya, the whole of the winter's production is consumed within a year, and the reason is evident; it is impossible that the repeated heatings which the sake requires during the summer months in order to prevent it going utterly to decay should be without effect upon its quality. Further the liquid is not heated until the brewer detects an incipient spoiling, which means the already considerable development of ferment with the production of butyric acid, and although a portion of the latter is probably driven away on heating, some is sure to remain. By repeated fermentation and heating, therefore, the amount of butyric constantly increases, and thus in time, the sake must become -undrinkable.
A process simple and effective, which will preserve the sake is evidently greatly desired by brewers, as is shown by the many attempts which have been made to use salicylic acid for this purpose. Mr. Korschelt wrote a pamphlet advocating the use of this antiseptic, and succeeded in persuading many large brewers to try it, but so far as I can learn, the success of the experiment has not been such as to satisfy the expectations raised. Salicylic acid has been introduced in Europe of late years as a means of preventing the deterioration of wine and beer, and when employed in sufficient quantity appears to answer the purpose in the climates of England and Germany. Prof. Kolbe mentions that "salicylic acid added to new wine entirely prevented after-fermentation. It appears also to prevent wine kept in half empty bottles becoming stale and sour. The quantity of the acid found sufficient for the purpose was 0.2 gram (or 0.1 gram salicylic acid and 0.1 gram acid potassium sulphate) per bottle." He also gives experiments showing the influence of the presence of differing quantities of salicylic acid upon a light, English beer, which would usually keep for about four months. The quantities added were to 100 liters of the beer.
The evidence of the experiments quoted above goes to show that when from 10 to 20 grams of salicylic acid are added to 100 liters of beer, or to about 110,000 grams, i.e., 1 or 2 in 10,000, the preservation is perfect during summers such as we are accustomed in Europe. How far the higher temperature experienced in this country will modify the results we have no means of knowing. The only direct experiments I am acquainted with, besides those of Mr. Korschelt, are mentioned by Prof. Kinch in the Transactions of the Asiatic Society of Japan.1 He says, "Numerous experiments were made last summer with salicylic acid as an antiseptic agent for sake, and it was found that used in the ratio of 1:10,000 it preserved sake in imperfectly closed vessels for about a month, and when used in the ratio of 1:5000 it preserved the sake through the whole of the summer even under very trying circumstances." This evidence corroborates that offered by Prof. Kolbe, and we must probably look to the quantities used by the brewers for an explanation of their want of success. One of their complaints was the expense of the material, and though I do not know in what proportions it was used, it may readily be imagined that they would err on the side of deficiency rather than on the opposite side.
Although the evidence in favor of the action of salicylic acid in arresting the change of alcoholic liquids, experiments have been conducted only for a comparatively short time, and there is nothing to show that the effect is a permanent one. Indeed from the chemical properties of salicylic acid, and especially from the readiness with which it is converted into salicylic ether in presence of alcohol and an acid, it may be regarded as certain that when a solution of the acid in sake is allowed to remain for a considerable time, especially at the summer temperature, it will be transformed into salicylic ether, and as this body probably does not possess the same antiseptic properties as the acid, the preservative effect of the acid will thus prove to be only temporary. Moreover the wood of the vessel in which such liquids are kept has been shown gradually to absorb the acid and thus destroy its utility. These circumstances will however, only necessitate the more frequent addition of salicylic acid, and as Prof. Kinch has shown that 1 part in 5000 of sake is sufficient to prevent the liquid spoiling during a whole summer it is only necessary that this amount should be added each spring the make the process successful. So long, however, as the price of salicylic acid is as high as it is at present in Japan, it will probably be more economical to heat the sake with such modifications in the form of the apparatus as will presently be described.
It is not necessary to wait until salicylic acid falls in price sufficiently to
make its use economical; the brewer has at hand all the appliances needful for m
aking his brew keep as long as he pleases, and without any additional expense fu
rther than that required to alter the shape of some of his vessels. I have point
ed out that the weak point of the present method is that the liquid after having
been heated is poured back into the same vessel in which it had formerly become
spoilt, and that the vessel is not completely filled. With the present form of vat used for storing sake it would be difficult, if not impossible, to completely fill it, and be sure that it was also perfectly tight, but if, instead of using the large, upright tuns which are covered by large, flat plates, 6 or 8 feet in diameter, and closed round the edges by means of paper and glue, a vessel were used with only a small bung hole at the upper side which would permit of being easily and securely fastened, the brewer need hardly wish for any other means of preserving sake. At present even at the largest brewery in Itami not much more than 1000 koku (180,000 liters) of sake are prepared in one season, but if proper means of preservation were employed, that amount might be largely increased, say to one million liters or 5500 koku. If the sake were distributed into small barrels holding, say 1 koku each, the number required in one brewery would not be greater than the space would admit of, with this advantage, that even if one barrel went bad the rest of the brew would not be affected. That the heating and preservation of the sake under the conditions mentioned above suffice to prevent the liquid spoiling has been shown by direct experiments with two sorts of sake, one from Itami, "Gaika", and the other from Nishinomyia, "Irozakari." Five bottles of each were heated in a vessel of water until the temperature of the contents rose to 60°C and were then tightly corked and sealed. At the end of twelve months the sake remained clear and brilliant, and had in no way deteriorated, whilst the same sake kept in a bottle closed in the ordinary way was completely spoilt, the change being indicated by the analyses in Table 27 and Table 28. This is evidence, although quite unnecessary, that the process applied to wines is likewise capable of application to sake.
An arrangement for heating sake which would be neither expensive to erect nor li
able to get out of order is represented in Figure
27, kindly furnished by Prof. Ewing. It consists of a long, wrought iro
n box (A), about six feet long, three feet deep, and three feet broad, made of b
oiler plate riveted together, and built over a small fireplace with flues circul
ating beneath and on both sides so that the whole of the vessel is pretty equall
y heated by the hot gases before they escape to the chimney. The right hand side
shows a section taken through the furnace some distance beyond the fireplace; t
he flue beneath is made broader than it would be at the fireplace and to prevent
the front part of the wrought iron vessel burning away too rapidly it would be
necessary to protect it from immediate contact with the flame by brickwork, whic
h, however, is not represented in the drawing, and need only extend a short dist
ance from the fireplace. In order to support the heating vessel it would be advisable to build up brick pillars in the middle of the flue, but it would not be necessary to make them very broad. The vessel is provided with a lid which can be removed when it is required to clean the inside; it has in the center a long opening, somewhat larger at one end, which is usually covered with wooden plates. The larger square opening (b) is for the introduction of the sake to be heated; the longer one for the purpose of stirring the liquid in order to equalize the temperature as much as possible. That there may be no danger of the iron becoming burnt by the exposure of its sides to the action of the hot air when there is no liquid within to protect it, it will always be found advisable to withdraw the fire from the grade before removing the heated sake. A vessel of the size given will hold about 8 koku of hot sake. To permit the withdrawal of the sake and its introduction into proper vessels which may be completely filled with it while still hot, a pipe is led through the brickwork and reaches some distance beyond it ending in a stopcock and curved neck, the vertical portion being made to slide up and down so that it may allow of the passage under it of a barrel in the way shown in the diagram. At the side of the furnace a depression in the ground is made in such a way that the barrel, resting upon a small barrow, can be wheeled down an inclined plane on one side and be brought right under the tap, and when filled can be pushed forward, and its place taken by a fresh one, and so on until the greater part of the liquid has been stored. As soon as the barrel is filled it is, of course, tightly closed in the usual way. The barrels which would be suitable for this purpose are such as are used in beer breweries, and some very good examples are shown by the Kai tak shi (Colonization Department) in the present National Exhibition (1881).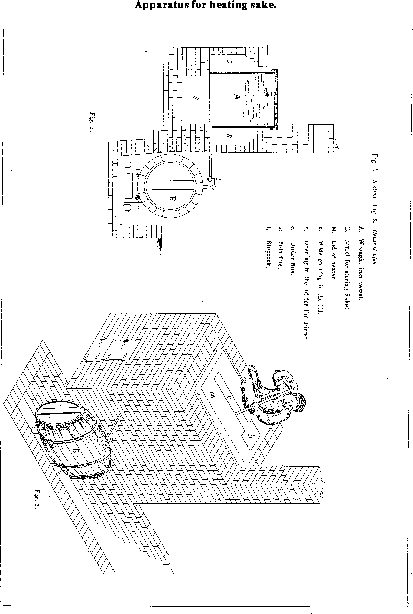
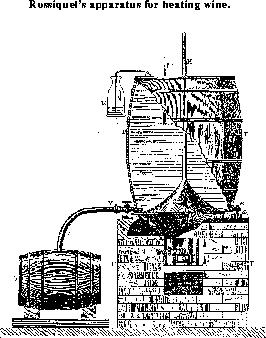
In Figure 28, a form of apparatus for heating wine, devised by M. Rossignol2 is shown, taken, by kind permission of the author, from M. Pasteur's work on wine, p. 232 (Ed. of 1873). The following is a translation of the description which accompanies the drawing. "This apparatus consists of three parts: 1.) a furnace F, which does not differ from any ordinary furnace; 2.) a broad, copper boiler C. provided with a cover soldered to it, and prolonged into a straight tube H, open at the end: the apparatus is filled with water half up the tube, and serves as a water bath; 3.) a wooden trough or barrel T, the bottom of which is awn off, and which rests upon the edge of the lid of the boiler and is firmly fastened to the cover by a simple arrangement: the edge of the cover a extends beyond the boiler for 3 or 4 centimeters; below it is a ring of wrought iron, and above a washer of caoutchouc, upon which rests the edge of the barrel; an iron ring encircles the edge of the barrel and is provided with straps of iron e which are fastened to the lower ring by strong bolts. The interval between the outside of the boiler and the inside of the barrel is filled with the wine, and all that portion of the boiler with which the wine comes in contact is tinned. A thermometer t indicates the temperature of the wine; a vessel E with tube allows the apparatus to be completely filled and the wine to expand on heating. A simple glance at the figure will explain how the apparatus works. It heats 6 hectoliters (3.3 koku) in 1 hour, uses 10 centimes (10 sen) of fuel per hectoliter, and costs 140 francs."
This apparatus like the one before mentioned, has the disadvantage of being intermittent. The following description applies to the apparatus of M. Terrel des Chenes, shown in Figure 29, also taken from M. Pasteur's "Etudes sur le vin", p. 245. Figure 30 shows the arrangement of casks and heating apparatus at work. The heat generator consists of:
- A central fire box F in the form of a truncated cone; the fire occupies the lower part. The fuel is introduced at first through the side opening P, and when the apparatus is at work, through the small door P' made in the chimney. A register moderates the draft.
- A water batch B, which occupies the whole of the interval between the fire box and the outer cylinder. v is a clearing cock. Above the bath is a reservoir open to the air, constantly full of water, separated from the water bath by means of a horizontal partition, and communicating with it by a valve o attached to a lever. The lever itself is connected with the stopcock v by means of a chain; when from any accidental cause the temperature of the bath rises too high, the vapor escapes through o, the water enters and the bath is brought back to the normal temperature and is fed at the same time. If, for any reason, the apparatus has to be stopped for any time and the temperature of the bath rises too much, the same result is attained by opening the stopcock v which raises the valve o, the cold water which is always kept in the open reservoir enters the bath and cools it.
- A worm ss, through which the wine flows; this consists of 40 small copper tubes, 4 millimeters in internal diameter, which open at one end at the mouth N, at the other at K, after having made nearly two turns in the water batch. The cooler RR is formed of a very large pipe surrounding the heat generator, containing inside 40 small parallel tubes s', 4 millimeters in diameter, like those within the bath. They open at one end into a box H, in which a thermometer dips to indicate the temperature, and at the opposite end of the wide tube into a cavity, R.
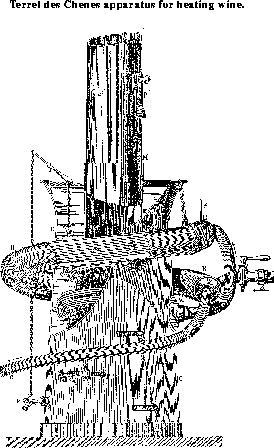
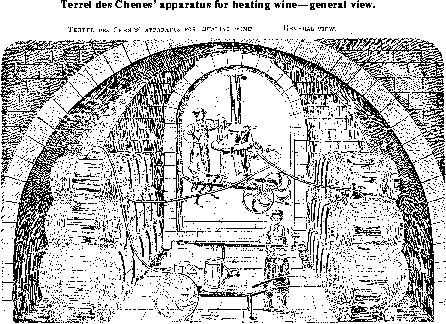
When in action the wine flows in the following way through the apparatus. The cold wine enters by the tube a into R in the wide gland which forms the cooler, circulates on the outside of the small tubes in RR; and leaves at N' by a tube passing at once into the heat generator; traverses the 40 tubes ss of the apparatus, leaves it at K and enters the cooler by the tube l, flows through the 40 small tubes s's' (cooled by the newly arrived cold wine) and finally leaves the heating apparatus by the tube e. Figure 30 presents a perspective view of the complete apparatus and the mode of using it. It is represented by B at the opening of the cellar; it is borne upon a barrow and may be moved by one man; an air pump A, also supported upon a barrow, is used to compress the air in the upper part of the cask T, the wine contained in which is to be heated; a pipe inserted in the lower part of the cask brings the wine to e in the heating apparatus B; another pipe S conducts the heated wine into an empty cask T'.
To set the whole at work the water bath is filled, the wine is forced into the apparatus by working the pump; and when the water is hot enough, the stopcock S is slightly opened: the thermometer rises; when it reaches 60°, for example, the stopcock is opened more, and then only is the wine received into the empty cask. One man works the pump, while another takes charge of the heating apparatus and regulates the flow of the wine by means of the stopcock, watching the thermometer all the time.
When, the operation ended, the apparatus has to be cleaned, the valve o is unscrewed, and in its place the extremity of the tube e is inserted; a current of stream then passes through the apparatus in a direction opposite to the flow of the wine, and drags away the deposit which has formed in the tubes.
The following data will given an idea of the economical results of this apparatus:
The large apparatus receiving the wine at 15°C raises it to 60° and cools it to 32°C. It requires 5 kilos of coal per hour, costing 1-1/2 centime per hectoliter; its diameter at the base is 0.50 meter, its total height 2 meters. The total weight with pump and other requisites does not exceed 230 kilos.
At present as the sake cannot be preserved without alteration for any length of time, the beneficial results of "ageing" have not been experienced, and a decided improvement in the quality of the liquid may be looked forward to by the adoption of the heating and preserving in well closed, wooden barrels. One effect would be that a quantity of air would diffuse through the wood and would mature the wine without the danger of any disease germs accompanying it. The influence of oxygen upon wine cannot be better described than in Pasteur's own words.3 "In my opinion it is oxygen which makes the wine; it is by its influence that the wine ages; it modifies the bitter constituents of new wine, and causes the bad taste to disappear; it is the same agent which induces the formation of deposits of good character in casks and in bottles, and far, indeed, from an absorption of a few cubic centimeters of oxygen per liter of wine spoiling it, removing from it its "bouquet" and weakening it, I believe that wine has not come to its proper state, and should not be bottled, so long as it has not absorbed an amount of oxygen much greater than that."
[Top] [Prev] [Next] [Bottom]
1
Vol. VIII, p.407.
2
I know, the figure says "Rossiquel". I think the original mss. has a typo. MAS
3
Etudes sur le vin. 1873, p. 85.
stevens@stsci.edu